Developmental vs Degenerative Genetic Diseases
 Genetic diseases can also be classified in terms of when they affect the animal. Developmental genetic diseases are often apparent soon after birth or in the young puppy. Degenerative genetic diseases affect the adult animal, either young adult or older animal.
Genetic diseases can also be classified in terms of when they affect the animal. Developmental genetic diseases are often apparent soon after birth or in the young puppy. Degenerative genetic diseases affect the adult animal, either young adult or older animal.
Genetic diseases that are apparent before the animal is of reproductive age are easier to control and eliminate from a breed because affected animals will not be used for reproduction. Genetic diseases that affect animals later in life, after the reproductive years, are much more difficult to control and eliminate since the bad genetics has often already been passed on to the next generation before the disease becomes apparent.
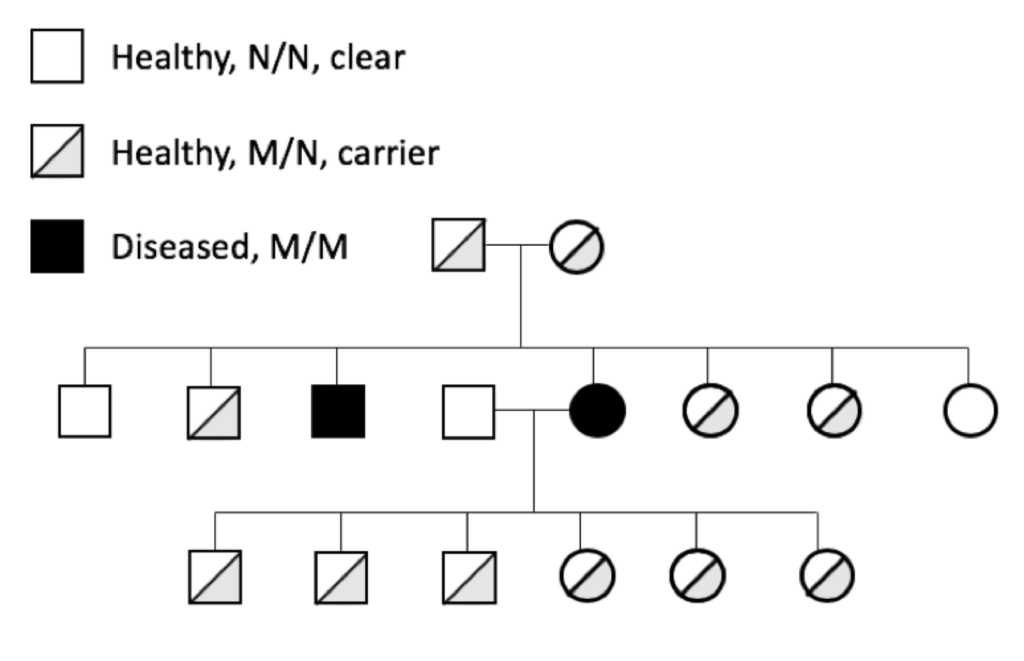
Genetic diseases can cause symptoms with a range of severity in the dog, from mild and controllable with medical treatment to severe and life-threatening. Silent carrier animals (M/N) for recessive genetic diseases are historically problematic because of the difficulty in identifying them. They lead a normal and healthy life, but when used for breeding can pass a copy of M to the next generation.
The Frequency of Genetic Diseases in Dogs
 It is true that simple genetic diseases are found at a high rate or frequency in our domestic dog populations, particularly within purebred dog breeds, compared to the frequencies of simple genetic diseases seen in humans.
It is true that simple genetic diseases are found at a high rate or frequency in our domestic dog populations, particularly within purebred dog breeds, compared to the frequencies of simple genetic diseases seen in humans.
An example of a common simple genetic disease in humans is cystic fibrosis, which is an autosomal recessive disease with a frequency of about 1 person affected in 3000 people and a carrier frequency of about 1 person in 25. This compares with simple genetic diseases within dog breeds where it is not uncommon to have 1 animal (or more) out of 100 being affected and a carrier frequency of 1 animal (or more) out of 10.
The reason for this difference in frequencies of genetic diseases lies in the simplified genomes seen in dogs, due in turn to the degree of inbreeding encountered in dog breeds. Inbreeding is introduced at several levels:
- The creation of modern purebred dogs over the last 100 to 150 years, with a formal definition of breed standards and involving intensive breeding from within a limited foundation stock. This is, by definition, inbreeding.
- Closed pedigree books, where parents must be registered before offspring can be registered. The breed becomes a closed genetic box which exacerbates inbreeding.
- The modern dog show competition, which is an arena for artificial selection whereby canine winners are favored to be the parents of the next generation of animals. With artificial selection, it is people (us) who decide which dogs will pass their genetics (both good and bad) to the next generation.
- The inequalities between male and female reproduction allows the winning male dog to spread his genetics (both good and bad) extensively. This is known as the “popular sire effect”.
Genetic Mutations
The nature of mutations deserves a few words. A genetic mutation refers to a change in the sequence of DNA. Mutations represent the natural variations found within our DNA that we need in order to survive, adapt and evolve as a species. Mutations combined with natural reproduction allow natural selection via “survival of the fittest” to occur.
A substitution mutation is a mutation involving the simple replacement of one letter for another at one of the 2.5 to 3 billion or so letters in the DNA text that makes up our (or a dog’s) genome. An insertion (or deletion) mutation is the addition (or deletion) of one or more letters within the text of our DNA. In each case, whether mutation by substitution, insertion or deletion, the DNA text gets changed and no longer tells quite the same story that it used to.
The change can be good, bad or indifferent (neutral) for the animal. The bad mutations are more numerous and would spell disaster except for the fact that Mother Nature has kept a genetic “Ace” up her sleeve, by giving us two copies of all of our genes. Yes, we have two parents for a reason; please refer to Dog Genetics 1.0: The Basics. And thank your parents. Now the potential disadvantage of the bad mutations is outweighed by the potential advantage of the good mutations. Genetic variations are good, as long as we have natural selection. But in domestic animals, we no longer have natural selection.

Several Examples
With this information as a background, let us now look at a few specific examples of diseases, chosen to illustrate patterns and principles of genetic diseases in dogs. For a comprehensive list of simple genetic diseases of the dog, with descriptions and references, please refer to Dog Genetic Disease Search.
PRA-Type-1 (Papillons)

Progressive Retinal Atrophy (PRA) refers to a group of genetic diseases affecting the retina and involving the progressive degeneration of the rod and cone light receptor cells.
Rod receptor cells detect weak light and are useful for night vision as well as for peripheral vision. Cone receptor cells detect colour vision and are useful for day vision. PRA-Type 1 of the Papillon is a recessive genetic disease of the retina where clinical signs first appear around the age of 4 to 6 years.
Clinical signs of PRA-Type-1
Because the rod receptor cells are the first to be affected, dogs that are double mutant (M/M) will start to lose their night and peripheral vision. The development of the disease is slow but progressive; eventually the cone receptor cells are affected and dogs now begin to lose their day vision. An affected dog can retain limited day vision for up to several years, but the final outcome is usually blindness.
 PRA-Type-1, a new mutation
PRA-Type-1, a new mutation
Papillons are a small spaniel described by the American Kennel Club as friendly, alert and happy. Although Papillons are an old breed, PRA-Type-1 of Papillons is a newly recognized disease, first described in the scientific literature in 1995.
It is an example of a genetic disease seen only in one breed. Genetically, PRA-Type-1 behaves as a typical autosomal recessive genetic disease, where dogs with the disease are M/M double mutant, and dogs without the disease are either N/N clear or M/N carrier.
Proactive Papillon Breed Clubs
 PRA-Type-1 is caused by a rather complicated mutation within the CNGB1 gene that is a combination of both a deletion and an insertion and which was described in 2013. The Papillon breed clubs in North America are particularly active in trying to remove the mutation from their breeding stock.
PRA-Type-1 is caused by a rather complicated mutation within the CNGB1 gene that is a combination of both a deletion and an insertion and which was described in 2013. The Papillon breed clubs in North America are particularly active in trying to remove the mutation from their breeding stock.
At Labgenvet, we perform our tests by DNA sequencing, making the N/N, M/N and M/M animals easy to identify. Since 2016, Labgenvet has tested over 80 samples for this disease sent in by Papillon breeders.
DNA Sequencing Profiles and Frequencies
Here are representative DNA sequencing profiles and the mutation frequencies seen at Labgenvet:
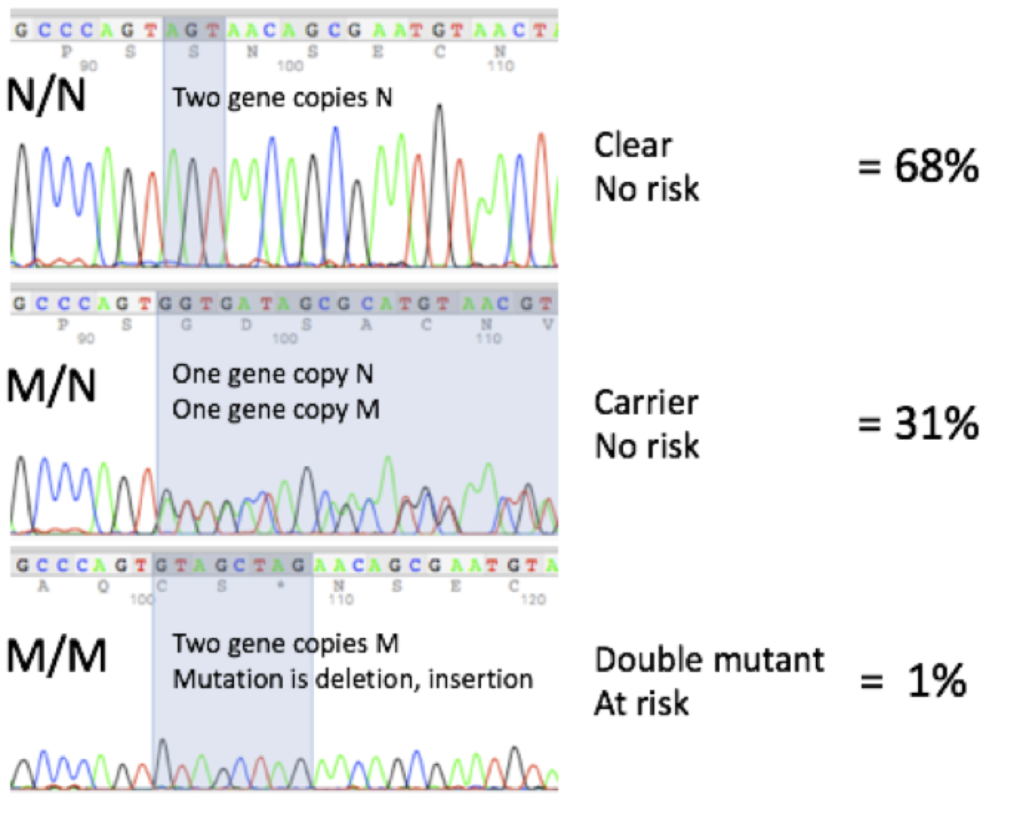
Although the probability is low of having an animal that is M/M double mutant at risk of having the retinal degeneration, the probability of having an animal that is M/N carrier is actually quite high. These probabilities will be reduced over time as the Papillon breeders continue to use the DNA test to help them with their mating decisions.
Multi-Drug Resistance (MDR1)
 Multi-Drug Resistance (MDR1) is a genetic condition wherein dogs that carry mutations in the ABCB1 gene (also called MDR1 gene) are hyper-sensitive to commonly used veterinary drugs; for this reason, it is considered a “pharmacologic” genetic disease.
Multi-Drug Resistance (MDR1) is a genetic condition wherein dogs that carry mutations in the ABCB1 gene (also called MDR1 gene) are hyper-sensitive to commonly used veterinary drugs; for this reason, it is considered a “pharmacologic” genetic disease.
The MDR1 gene codes for a transporter protein that protects the brain from toxic small molecules by binding and transporting the molecules out of the brain. A dog with two mutated copies of the MDR1 gene and thus no functional transporter protein is particularly sensitive to toxicity from a range of common drugs used in veterinary medicine including antiparasitic drugs (macrocyclic lactones such as Ivermectin), antibiotics (such as Erythromycin), tranquilizers (such as Acepromazine), antidiarrheal drugs (such as Loperamide), opioids (such as Butorphanol), and other drugs including cancer chemotherapy drugs (such as Vincristine, Vinblastine, Doxorubicin).
Clinical Signs of MDR1
Signs of toxicity can include loss of alertness, excessive salivation, pupil dilation, tremors, ataxia, seizures, slowed heart rate, coma, respiratory arrest and death. Note that signs of toxicity are much more severe for the animal that is double mutant but have been reported for animals that carry a single mutation. For this reason, the heredity of Multi-Drug Resistance is considered as dominant with variable penetrance, and although the double mutant (M/M) animal is most at risk of showing severe toxicity to medications, care should still be taken when giving medication to carrier (M/N) animals.
 MDR1 and the Shepherd Breeds
MDR1 and the Shepherd Breeds
The mutation for MDR1 is found in a number of dog breeds, mostly of the Shepherd and Collie-type or their crosses. These breeds include: Australian Cattle Dog, Australian Shepherd, Berger Picard, Border Collie, Chinook, Collie, German Shepherd, Long-Haired Whippet, McNab Shepherd, Miniature American Shepherd, Miniature Australian Shepherd, Old English Sheepdog, Rough Collie, Ryukyu Inu, Shetland Sheepdog, Shiloh Shepherd, Silken Windhound, Smooth Collie, Waller, White Swiss Shepherd.
This list of breeds indicates that the mutation that is the cause of the problem is fairly old, and happened before the Shepherd-type breeds were segregated into their present form.
Medication Intolerance
 The mutation that is the cause of MDR1 is a deletion of four bases within the ABCB1 gene. In order for toxicity to occur, dogs have to be given a pharmacological product. Dogs that are clear (N/N) for the mutation will have normal, wild-type tolerance to medications. Dogs that have one normal and one mutated copy of the mutation (M/N) are felt to have somewhat reduced tolerance (i.e. somewhat increased toxicity) to medications.
The mutation that is the cause of MDR1 is a deletion of four bases within the ABCB1 gene. In order for toxicity to occur, dogs have to be given a pharmacological product. Dogs that are clear (N/N) for the mutation will have normal, wild-type tolerance to medications. Dogs that have one normal and one mutated copy of the mutation (M/N) are felt to have somewhat reduced tolerance (i.e. somewhat increased toxicity) to medications.
The real clinical problem and the animal to avoid giving medication to is the double mutant (M/M) animal, for which otherwise safe doses of medication become highly toxic. The mutation was reported in 2001, so breeders and veterinarians have had access to DNA tests to identify carriers and animals at risk for almost twenty years. Despite this fact, M/N and M/M animals are still being identified.
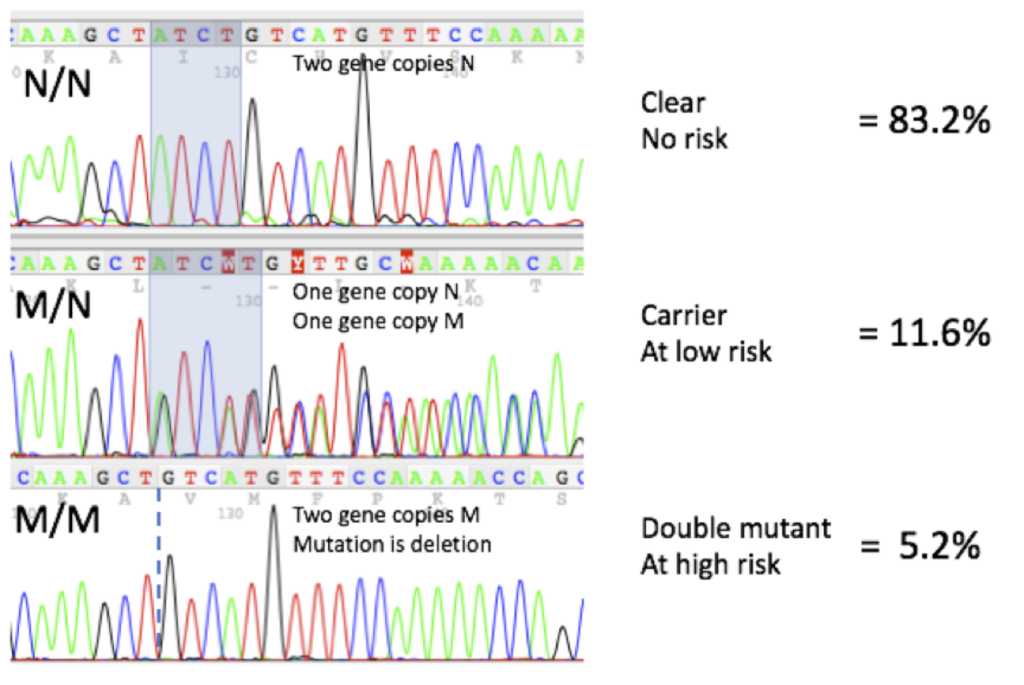
DNA Sequencing Profiles and Frequencies
Here are representative DNA sequencing profiles and mutation frequencies seen on more than 400 samples at Labgenvet:
Degenerative Myelopathy

German Shepherd
Degenerative Myelopathy (DM) is a neuro-muscular degenerative disease caused by a specific gene mutation that has been identified in many dog breeds. Additional genetic factors as well as environmental factors can also contribute to the time of onset and progression of the disease. DM is a disease that affects the white matter of the spinal cord and is equivalent to Amyotrophic Lateral Sclerosis (ALS; also known as Lou Gehrig’s disease) in humans.
Clinical symptoms of degenerative myelopathy
Affected dogs usually start showing symptoms of progressive muscular atrophy by 7 to 9 years of age, with initial loss of coordination of the hind limbs. These dogs can lose mobility six months to two years after the onset of clinical signs, with large dogs showing a more rapid progression of symptoms than small dogs.
Pain is not associated with the disease, and symptoms can progress to the point that the animal is incontinent and eventually paraplegic. There is no treatment and often the affected animal is euthanized for humanitarian reasons before these final stages.
The Genetics of Degenerative Myelopathy

Bernese Mountain Dog
DM in dogs is an example of a genetic disease that follows (more or less) simple or mendelian genetics. The DNA mutation that has been linked to DM is a simple substitution of one letter for another, found in the SOD1 gene. This mutation has been identified in over 180 dog breeds, so I will not list them all.
DM is considered an autosomal recessive disease with variable penetrance. Dogs from several breeds are particularly at risk of developing DM when the double mutation (M/M) is present in an animal. These breeds include (among others) the German Shepherd, the Bernese Mountain Dog and the Boxer.
Modifying Genes for Degenerative Myelopathy

Corgi
For some dog breeds, dogs can be M/M for the mutation in the SOD1 gene, but whether or not they develop DM now depends on additional genetic factors known as modifying genes.
A case in point is the Pembrooke Welsh Corgi. Corgis that are M/M for the mutation of the SOD1 gene can show clinical signs of DM by the age of 7 to 9 years, or can be free of signs at 15 years of age. The increased susceptibility seems to be due to the presence of a mutation within a modifying gene, and this modifying gene for Corgis has been identified. Unfortunately, modifying genes for other breeds, even if they are suspected, are not as yet identified.
The Bernese Mountain Dog is another breed worth mentioning. In this breed, a second mutation within the SOD1 gene was identified that can cause increased risk of having DM. This second mutation is not seen in other breeds. So simple genetics isn’t always so simple. The known genetics of DM in the dog pales in comparison to the known genetics of ALS in humans, where there are over 150 mutations documented within the human SOD1 gene, and at least 5 different modifying genes for ALS that have been identified.
A Recessive Disease Occurring Later in Life
There are several practical aspects concerning DM in dogs that are worth mentioning. It is a recessive disease that occurs later on in life, after the reproductive years of the animal. Of course, the M/M double mutant animals showing symptoms are a problem. But an additional problem, particularly with respect to controlling the disease within a breed, are the unidentified carrier (M/N) animals.
If M/N animals are unknowingly used for reproduction, there is a good chance that the mutation will be passed on to the next generation. More problematic, if two M/N animals are bred together, there is a 1 in four chance of producing M/M double mutant puppies at risk of developing DM later in their lives. These M/M puppies exist because I have seen their DNA; I know that at the age of 7 to 9 years, they will be taken into a veterinary clinic because they are starting to get wobbly in the back end. And the disease cycle continues.
Identifying Carrier Animals

Boxer
Although unidentified carrier animals are a problem, as soon as carrier animals are identified (usually by a DNA test), M/N animals are much less problematic. Carrier animals are not at risk of developing DM, and if bred to N/N clear animals there is no risk of producing M/M double mutant puppies. As I like to tell breeders, identifying a carrier (M/N) animal is good news, but of course the breeders would have preferred to have identified a clear (N/N) animal.
Be that as it may, due to the numbers of breeds of dog that have the SOD1 mutation, the frequencies of the mutation seen within breeds, the late age for disease onset, the rather complicated “simple” genetics of the disease as well as the decentralized nature of dog breeding, it will take many years before DM and its mutation are eliminated from our dog breeds.
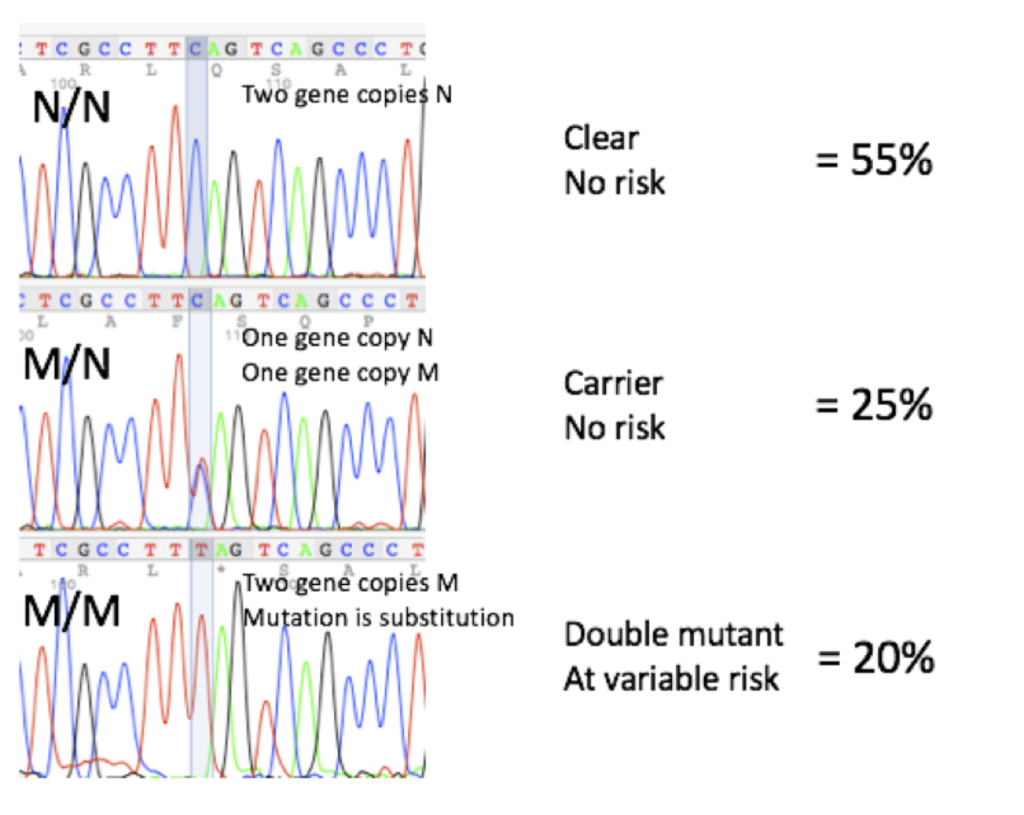
DNA Sequencing Profiles and Frequencies
DNA sequencing profiles and frequencies of animals that are N/N (clear), M/N (carrier) and M/M (double mutant) for the DM mutation, based on over 600 dogs tested at Labgenvet, are as follows:
For additional genetic information and references regarding Degenerative Myelopathy in dogs, please refer to the following link: Degenerative Myelopathy in dogs.
A comprehensive list of simple genetic diseases in dogs can be found at: Dog Genetic Disease Search.
Knowledge is Power, and Power is Responsibility

Our knowledge of the mutational basis of simple genetic diseases in dogs is increasing every year. With good, validated DNA tests we now have the power to identify carrier (M/N) animals.
We can, in theory, eliminate a simple genetic disease from a given breed within one breeding generation and eliminate the disease-causing mutation within two generations. The challenge is to take on the responsibility.
Genetic Diseases Are Not Always Simple

What I have presented so far is valid for simple or mendelian genetics, where one gene is involved and one mutation causes a disease. But, there are over 20,000 protein-coding genes in the dog’s genome, and genes don’t work in isolation. Complex genetic diseases, involving the contributions of mutations in multiple genes as well as environmental influences, do not follow the simple genetic rules we have just talked about.
Hip dysplasia in the dog is an example of a complex genetic disease, with multiple (as yet undetermined) genetic inputs plus a big dose of environmental influence. Health problems that are associated with exaggerated conformations (short face, short legs, short tail, long back, floppy ears, the list goes on…) are in fact genetic diseases that are caught up in the morass of breed conformation standards. Life and genetics are not always simple, even though we wish that they were.
© 2018 David W. Silversides
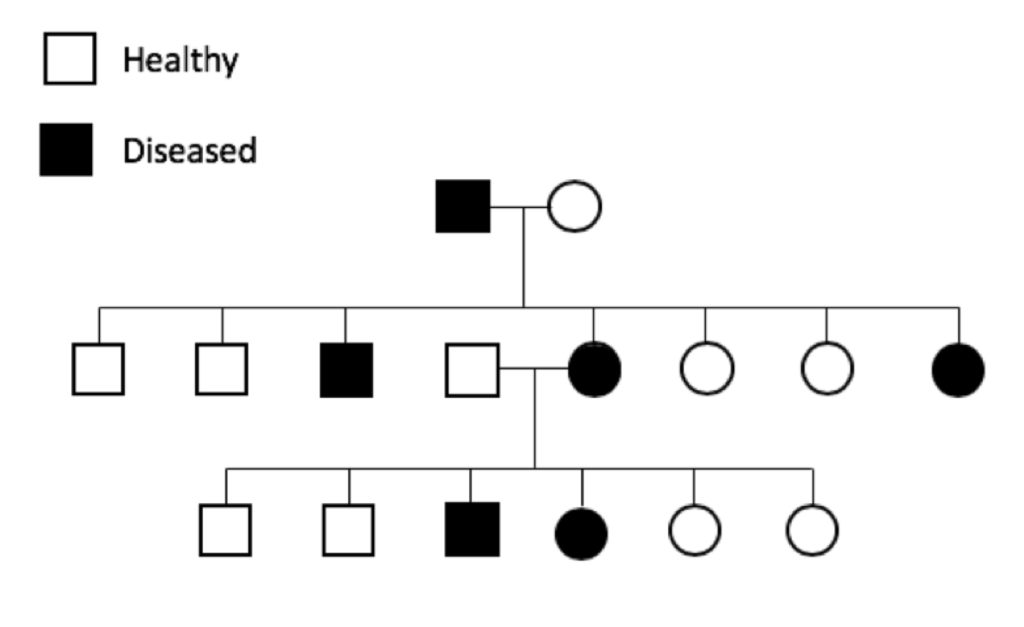
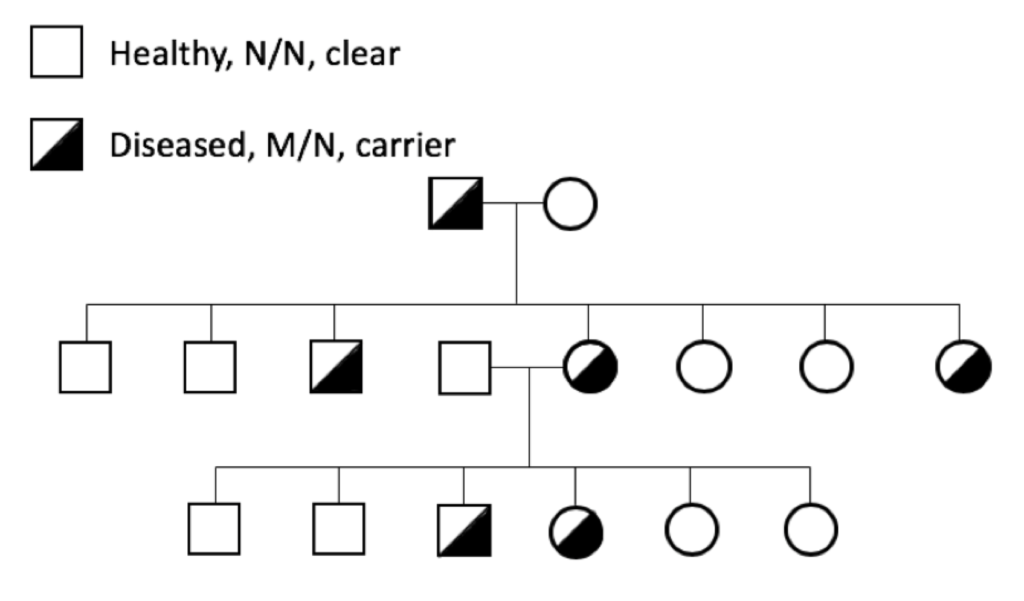
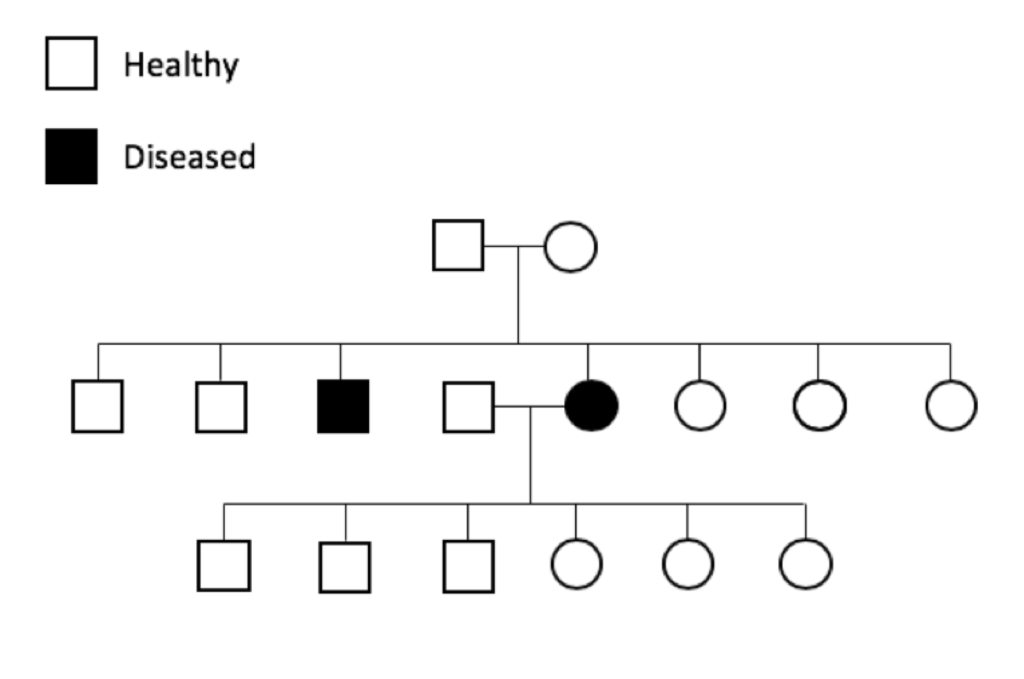
 The prevalence of genetic diseases in our dogs is a recognized and long-standing problem, and the problem is twofold. First and obviously, we have dogs that are sick because of a genetic disease. Secondly and more insidiously, we have dogs that are perfectly healthy but who are carriers for the disease-causing mutation.
The prevalence of genetic diseases in our dogs is a recognized and long-standing problem, and the problem is twofold. First and obviously, we have dogs that are sick because of a genetic disease. Secondly and more insidiously, we have dogs that are perfectly healthy but who are carriers for the disease-causing mutation. which the gene and the disease-causing mutation have been identified. For many mutations, the genetic disease is restricted to one breed; these tend to be recent mutations, occurring after the breed was established (generally in the last 100 to 150 years).
which the gene and the disease-causing mutation have been identified. For many mutations, the genetic disease is restricted to one breed; these tend to be recent mutations, occurring after the breed was established (generally in the last 100 to 150 years).